

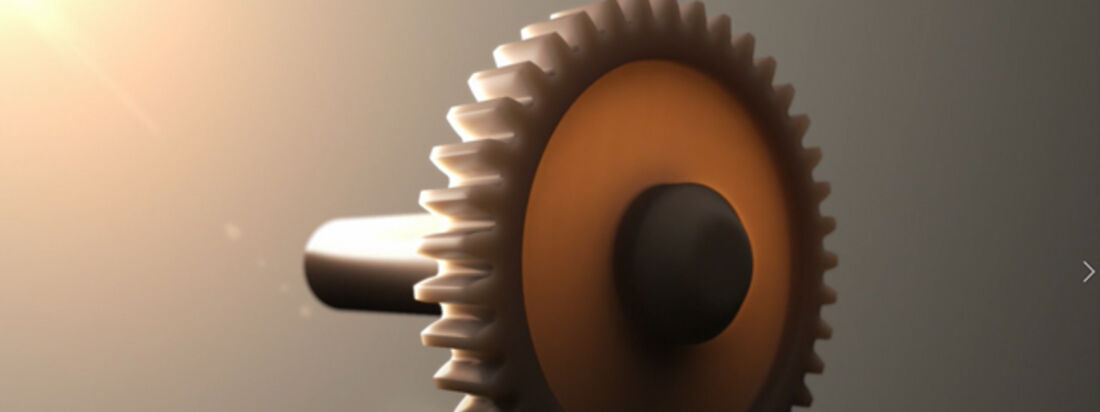
VESTAKEEP® FOR PLASTIC GEARS
Plastic gears are superior to metal gears in many aspects and are conquering new applications thanks to temperature-resistant high-performance plastics such as VESTAKEEP® PEEK. ... MORE

VESTAKEEP® ESD PEEK
Small electronic devices require narrower semiconductor fabrication. They need to occupy smaller spaces and perform better. But this leads to a critical challenge in processing: damage from electrostatic discharges (ESDs). ... MORE
Evonik's PLASTICS DATABASE
We have the right materials to
take you to the top! ... MORE





